Perry Baromedical's Hyperbaric Systems are FDA approved for over 60 years

Perry Baromedical's Hyperbaric Systems are FDA approved for over 60 years


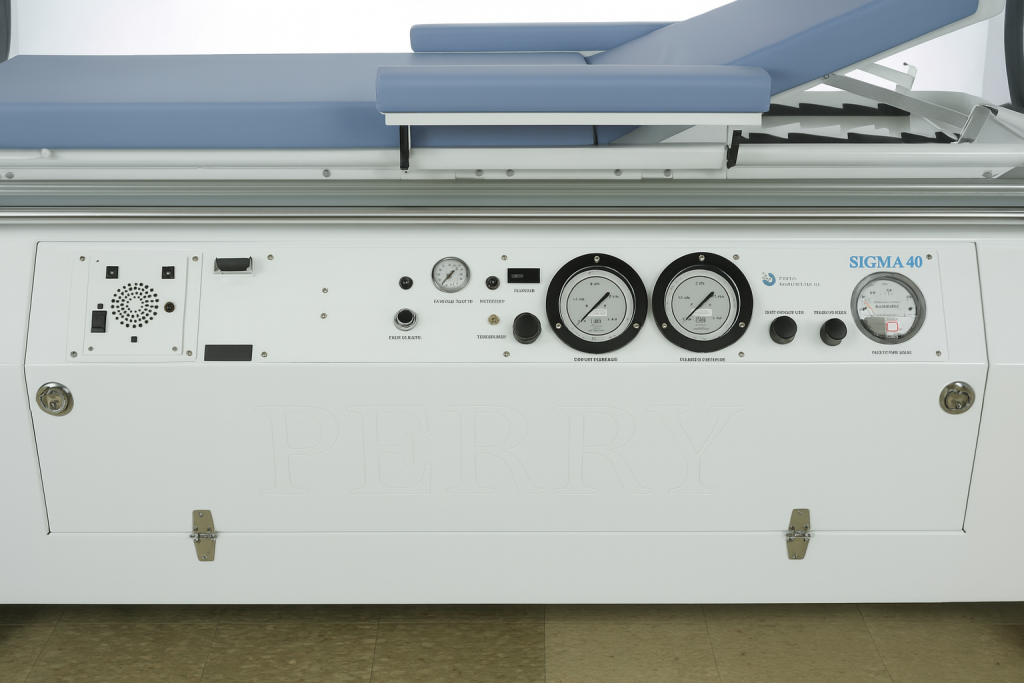
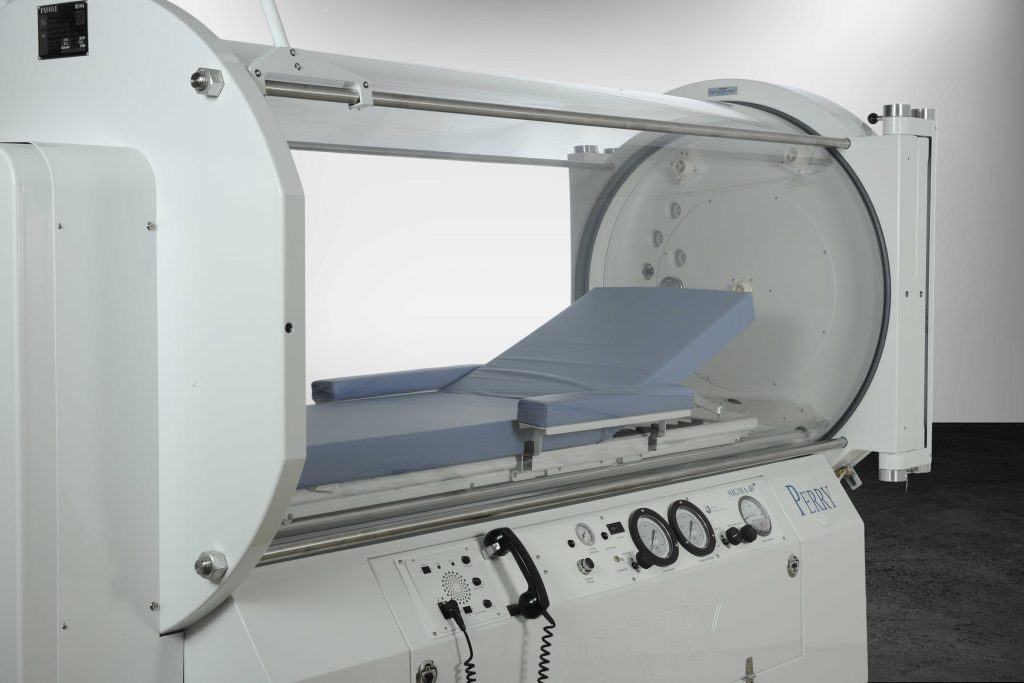
Perry Baromedical has been a distinguished manufacturer of registered medical devices for over 60 years, recognized for its unwavering commitment to excellence. As an ISO 13485:2016 certified leader in the industry, Perry specializes in the manufacturing, installation, and servicing of hyperbaric oxygen therapy systems specifically designed for medical use.
Perry’s hyperbaric oxygen chambers are classified as Class IIb medical devices by the U.S. Food and Drug Administration (FDA) and are subject to strict federal regulations.
All Perry systems are FDA approved and are meticulously designed, manufactured, tested, and installed in accordance with the latest FDA regulations, the ASME/PVHO-1, PVHO-2 codes, the Pressure Equipment Directive (CE Mark), the Medical Device Regulation (CE Mark), and the standards established by the National Fire Protection Association (NFPA 99).
Perry Baromedical is a distinguished and internationally recognized brand name, with a history of more than 60 years of design innovation and quality manufacturing in its field. Perry is the only FDA approve full-line manufacturer of hyperbaric chambers in the industry, with its product line encompassing monoplace, dualplace, and multiplace systems. The Company sells its products to hospitals, private clinics, medical teaching institutions, critical access and children’s hospitals, and burn institutes across the globe. Perry leads the HBO industry with a reputation for doing business with integrity and providing products of superior quality.
In its present form, Perry Baromedical is a young company. But its roots go back to 1956. We are proud of that heritage, and of the many milestones achieved.